Vs.
Venus Versa™
Multi-Treatment Platform
A versatile multi-application platform that provides the most popular, non-invasive aesthetic procedures, including photorejuvenation / photofacial, acne treatment, hair removal, skin resurfacing and wrinkle reduction.
TriBella™
TriBella™ is a complete skin rejuvenation treatment, which combines three effective, unique technologies into one treatment protocol to improve tone, tightness, and texture. Exclusively available with our Venus Versa™ system, TriBella™ treatments give clients a more youthful appearance.
Target Tone, Texture, & Tightness



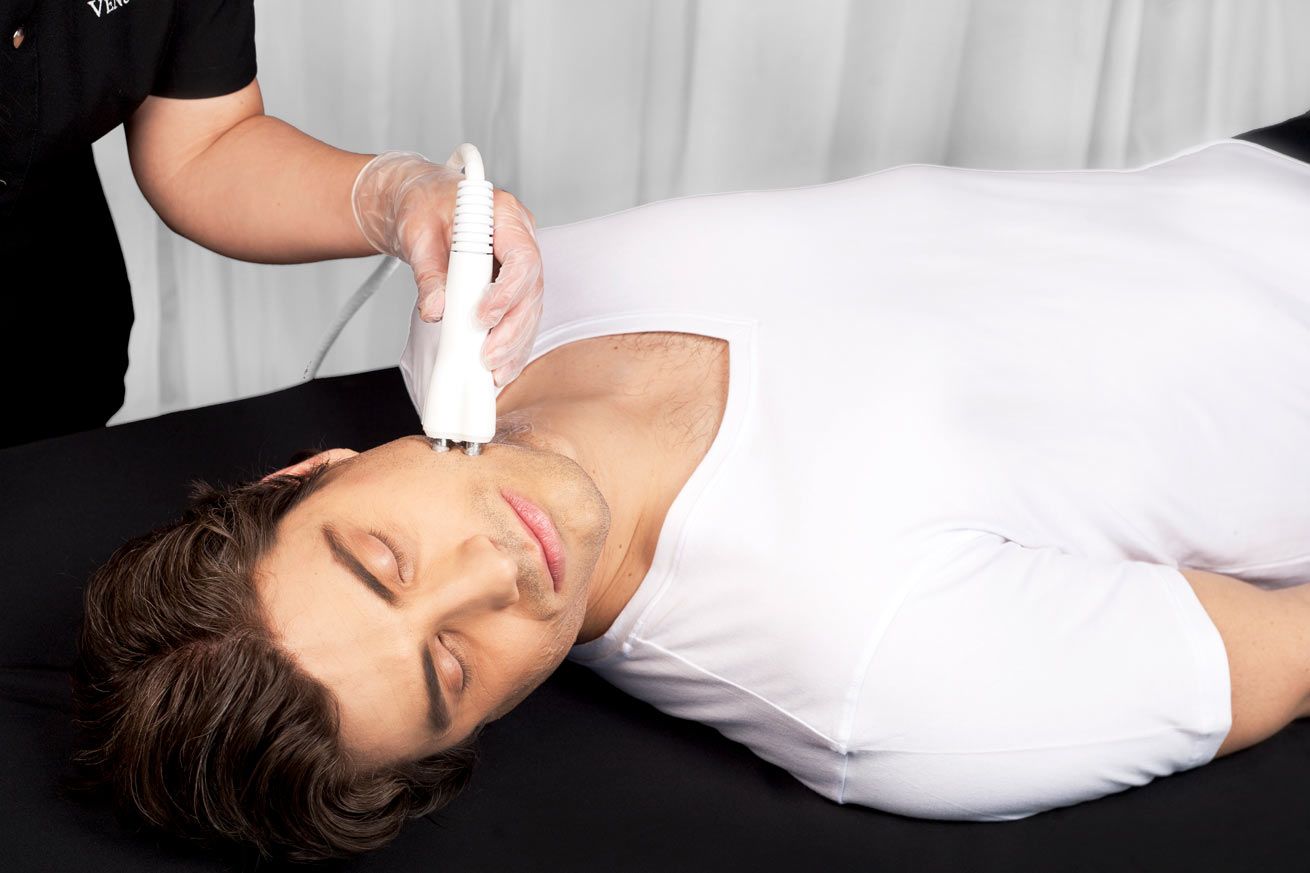
Skin Resurfacing with NanoFractional Radio Frequency
The Venus Viva™ MD applicator is is a highly customizable skin resurfacing technology with low downtime and is safe for all Fitzpatrick skin types.
-
MORE
- NanoFractional skin resurfacing allows the operator to customize ablation and coagulation settings. Radio frequency energy is delivered into the skin, creating micro-channels surrounded by intact tissue, which promote faster wound healing, little-to-no downtime, and reduces the risk of post-inflammatory hyperpigmentation (PIH).
- SmartScan™ technology uses a unique algorithm and pattern selection technology that enables the operator to generate customized patterns for maximum flexibility and patient safety.
- Improves the appearance of textural skin conditions such as scars (including acne scars), rosacea, striae, uneven skin texture, enlarged pores, dyschromia, and pigmentation.
Intense Pulsed Light (IPL) Technology with SmartPulse™
The IPL photorejuvenation, acne treatment, and hair removal applicators include innovative SmartPulse™ technology to ensure comfortable, precise, and consistent energy delivery through advanced pulse optimization. Real-time cooling system monitors the light-guide temperature 1,000 times a second for unparalleled patient safety and comfort.
-
MORE
- Photorejuvenation/Photofacial: Effectively target & treat vascular and pigmented lesions on the skin.
- Hair Removal: Efficiently target melanin in the hair shaft for comfortable hair removal treatments.
- Dual-light-guide Acne Treatment: Innovative dual-light-guide technology: blue light targets porphyrin caused by Propionibacterium Acnes bacteria, leading to the destruction of the P. Acnes bacteria, while the red light promotes faster healing and effectively decreases inflammation.
Multi-Polar Radio Frequency
For wrinkle reduction:
Unique combination of Multi-Polar Radio Frequency (RF) and Pulsed Electro Magnetic Fields (PEMF).
-
MORE
- With the capabilities of multi-polar radio frequency applicators, the Venus Versa™ uses a complex algorithm to deliver homogeneous energy and volumetric heating to multiple tissue depths, allowing for quick and safe buildup of heat and easy maintenance of therapeutic temperature throughout the treatment
- Effect of Multi-Polar RF is enhanced by PEMF, a non-thermal mechanism emitted through the applicator’s electrodes
- Through synergistic (MP)², RF heats and directly stimulates fibroblasts, while PEMF is known to promote angiogenesis and induce fibroblast proliferation through release of the growth factor FGF-2, resulting in increased collagen synthesis²
- (MP)² is therefore effective in collagen remodeling for wrinkle reduction, and the creation of new capillaries for renewal of blood supply.
Venus Versa™ offers three unique technologies: Intense Pulsed Light (IPL) with SmartPulse™, NanoFractional Radio Frequency with SmartScan™, and Venus proprietary (MP) 2 technology (Multi-Polar Radio Frequency + Pulsed Electro Magnetic Fields).
With the ability to meet today’s most in demand aesthetic treatments the Venus Versa™ is a sound investment for any clinic looking to expand its offerings with one workstation. Our versatile multifunction machine allows you to offer a variety of services, increase patient demand and positively impact your ROI – all factors that will help your practice thrive for years to come.
TECHNOLOGY
Photorejuvenation

The photorejuvenation protocol in the TriBella™ treatment corrects benign pigmented lesions and benign vascular lesions.
Wrinkle Reduction
Enhances collagen production to reduce the appearance of skin laxity, wrinkles and rhytides.
Skin Resurfacing

Improves the appearance of textural skin conditions such as scars (including acne scars), rosacea, striae, uneven skin texture, enlarged pores, dyschromia, and pigmentation.
The Results Speak for Themselves
All of the before-and-after photos for our aesthetic treatments are from certified Venus providers showcasing real patient results. Images are never edited or altered.
Tech Specs
Technology
-
AC DUAL IPL APPLICATOR
Wavelength - 415-480 nm, 630-950 nm
Optical Fluence - 5-25 J/cm2
Pulse Duration - 10-20 ms
Spot Size - 10 mm x 30 mm
Pulse Repetition Rate - Up to 3 Hz
-
NANOFRACTIONAL RF™ APPLICATOR
Output RF Frequency - 0.46 MHz
Fuse -3.15A, 250V
Max. Output Energy - 62 mJ/pin
Ablation Depth - Up to 500 microns
Number of Pins - Up to 160
-
DIAMONDPOLAR™ & OCTIPOLAR™ APPLICATORS
Output RF Frequency - 1MHz
RF Output Power- 0-150 Watts
Number of (MP)2 Synthesizers - Up to 8
Magnetic Pulse Frequency - 15 Hz
Pulsed Magnetic Field - Up to 15 Gauss
-
Device
Electrical Requirements - 100-240 VAC ,12.5A, 50-60Hz, Single Phase
Dimensions - 55 cm x 65 cm x 110 cm / 21.7 in x 25.6 in x 43.3 in (D x W x H)
Weight - 35 Kg / 77.2 lb
-
SR515 / SR580 IPL APPLICATORS
Wavelength - 515-950 nm, 580-950 nm
Optical Fluence - 5-25 J/cm2
Pulse Duration - 10-20 ms
Spot Size - 10 mm x 30 mm
Pulse Repetition Rate - Up to 3 Hz
-
HR650 (XL) / HR690 (XL) IPL APPLICATORS
Wavelength - 650-950 nm, 690-950 nm
Optical Fluence - 5-20 J/cm2
Pulse Duration - 20-50 ms
Spot Size - 10 mm x 30 mm (XL: 20 mm x 30 mm)
Pulse Repetition Rate - Up to 3 Hz

“The Venus Versa™ platform technology provides the best combination of all. With about 10 different handpieces, each for different procedures, I, as the physician, can choose which combination is best suited to my practice, my patients. I selected Venus Versa™ with Intense Pulsed Light for hair removal and skin rejuvenation.”
Robert M. Freund, MD
Speak To An Expert
To book a consultation or a demo, please contact us by clicking the button below and a representative will reach out to you directly.
INDICATIONS FOR USE:
Venus Versa™ is cleared by the FDA, licensed by Health Canada, and has CE Mark as a multi-application device intended to be used in aesthetic and cosmetic procedures. The SR515 and SR580 applicators are cleared by the FDA, licensed by Health Canada, and have CE Mark for the treatment of benign pigmented epidermal and cutaneous lesions and treatment of benign cutaneous vascular lesions. The HR650/HR650XL and HR690/HR690XL applicators are cleared by the FDA, licensed by Health Canada, and have CE Mark for the removal of unwanted hair and to effect stable long-term or permanent hair reduction for Fitzpatrick skin types I-IV. The AC Dual applicator is cleared by the FDA, licensed by Health Canada, and has CE Mark for the treatment of acne vulgaris. The DiamondPolar™ and OctiPolar™ applicators on the Venus Versa™ system are cleared by the FDA for non-invasive treatment of moderate to severe facial wrinkles and rhytides on females with Fitzpatrick skin types I-IV. The DiamondPolar™ applicator is licensed by Health Canada and has CE Mark for non-invasive treatment of moderate to severe facial wrinkles and rhytides on females with Fitzpatrick skin types I-IV. The OctiPolar™ applicator on the Venus Versa™ system is licensed by Health Canada and has CE Mark for temporary body contouring via skin tightening, circumferential reduction, and cellulite reduction. The NanoFractional RF™ (Viva) applicator is cleared by the FDA, licensed by Health Canada, and has CE Mark for dermatological procedures requiring ablation and resurfacing of the skin.
REFERENCES:
Ponqgsrihadulchai N, Chalermchai T, Ophaswongse S, Pongsawat S, Udompataikul M. An efficacy and safety of nanofractional radiofrequency for the treatment of striae alba. J Cosmet Dermatol. 2016; 1-7.↩
Callaghan, M. J., Chang, E. I., Seiser, N., Aarabi, S., Ghali, S., Kinnucan, E. R., . . . Gurtner, G. C. (2008). Pulsed Electromagnetic Fields Accelerate Normal and Diabetic Wound Healing by Increasing Endog







